Business Areas
30 Respiratory
30 Respiratory is our innovative respiratory disease platform consisting of a range of inhaled assets based on our proprietary nitric oxide-generating technology.
These inhaled products are far more potent against even highly drug resistant bacteria than the current standard of care antibiotics and combine this outstanding activity with an extremely broad spectrum of action and superb safety profile.
RESP301
Our lead asset, RESP301, is our breakthrough NO-generating respiratory product that both directly kills viruses and bacteria and boosts the patients’ immune response against these microbes.
RESP301 has demonstrated compelling safety and tolerability in human clinical studies and is in multiple ongoing Phase II clinical trials including in cystic fibrosis patients with Mycobacterium abscessus infections, a condition for which there are no approved therapies.
RESP30X
We have built on our experience creating RESP301 to develop a new portfolio of more than 10 inhaled respiratory antimicrobials which leverage our unique platform technology to deliver much higher payloads with the same excellent safety and toxicological profile of RESP301. They have been designed for a broad range of different indications and will complete Phase I studies later this year.
Tuberculosis
Tuberculosis (TB) infects 10.6 million people annually and is responsible for over 1.6 million deaths. This devastating burden of disease is growing year on year and disproportionately affects patients in developing countries. In response, we have commenced a program to develop anti-TB therapies and have completed a comprehensive preclinical program. Our lead candidate is planned to enter Phase II early bactericidal activity studies in newly diagnosed TB patients in October this year.
30 Therapeutics
30 Therapeutics is the division focused on developing novel treatments powered by our core technology that can be used to treat a wide range of human diseases.
Rare diseases
An absolute or functional deficiency of nitric oxide plays a critical role in several diseases that, whilst severe, affect smaller populations. Our platform technology shows promise both as a means of returning tissue NO back to physiological levels and to deliver broad spectrum antimicrobial activity.
Oral health
An ageing population and increased awareness of the profound impacts of poor oral health have driven an explosion in the number and complexity of oral surgeries undertaken every year. Many of these are complicated by infection which leads to serious long-term consequences. Unfortunately, the mouth represents a unique challenge for conventional antibiotics being both polymicrobial and difficult to penetrate. 30 Technology is ideally positioned to develop a range of solutions that can prevent or treat these surgical and implant related infections
Ophthalmology
Nitric oxide is intimately involved in regulation of infection and ocular pressure. We are actively exploring utilising its technology in topical formulations that can be used in a wide variety of infectious and pathological conditions.
30 Animal Health
We are looking at a range of ways of applying the 30 Technology platform and applying to animal health.
The initial focus is on taking advancements made by our respiratory platform to be adapted for use in animals. This includes wound healing and the treatment of livestock.
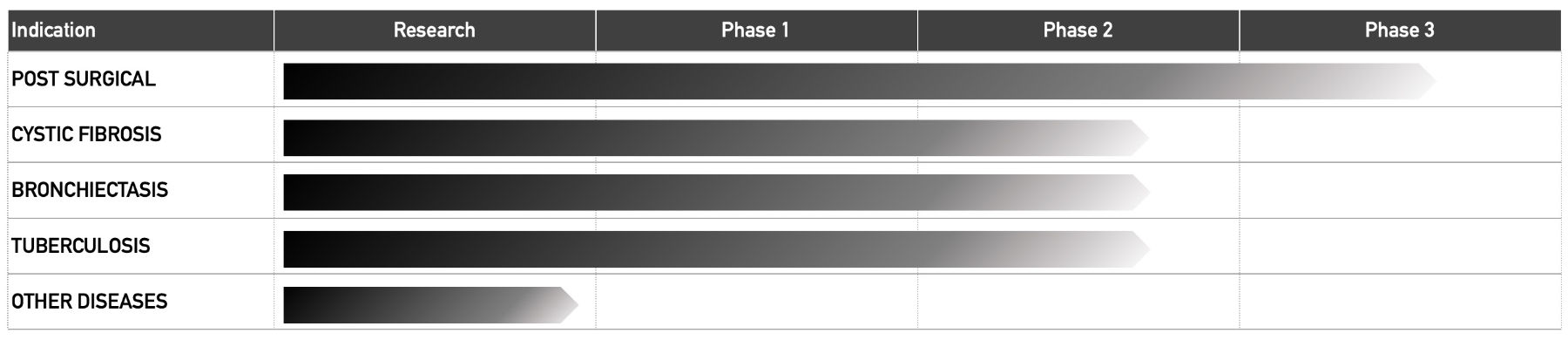
Pipeline
30 Technology’s NO-generating platform is being investigated in a range of disease areas, outlined below.
30 Technology’s lead programme is RESP301, a NO-generating breakthrough respiratory product, with clinical trials ongoing in cystic fibrosis patients with Mycobacterium abscessus infections.
Clinical Programme: Chronic Respiratory Disease, Bacterial & Viral infections